Formaldehyde
Contents |
[edit] What is formaldehyde?
Formaldehyde (HCHO, CH2O, or H2CO) is a colourless, strong-smelling, flammable chemical produced industrially, although in small amounts it occurs naturally through the chemical processes of living organisms and through the decay of plant material in soil. It is considered to be carcinogenic to humans based on evidence that it can cause nasopharyngeal cancer and leukaemia. As of 2016, it was classified as a restricted category 1B carcinogen in the UK, requiring suppliers to be registered.
It is an aldehyde or is classified in the formyl group; it is also known as formalin, methanal, methylene oxide, oxymethyline, methylaldehyde, or oxomethane, and has many other different derivatives. It is used in the production of phenolic, urea,melamine, and polyacetal resins. The most common names refer to the joint names: urea formaldehyde(UF), phenol formaldehyde (PF), and melamine formaldehyde (MF), as well as polyacetal resins, pentaerythritol, 1,4-butanediol, methylenebis, hexamethylenetetramine (HMTA), and some others. It may also be a by product of chemical reactions with formaldehyde-releasing preservatives like bronopol.
Formaldehyde is one of the most well-known volatile organic compounds, or VOCs. Organic compounds are defined in The Volatile Organic Compounds in Paints, Varnishes and Vehicle Refinishing Products Regulations 2012 as 'any compound containing at least the element carbon and one or more of hydrogen, oxygen, sulphur, phosphorus, silicon, nitrogen, or a halogen, with the exception of carbon oxides, inorganic carbonates, and bicarbonates', with a volatile organic compound being 'any organic compound having an initial boiling point less than or equal to 250°C measured at a standard pressure of 101.3 kPa'
Formaldehyde was accidentally produced in the mid-1800s by Alexander Mikhailovich Butlerov and discovered by A. W. Hofmann in 1868, who passed a mixture of methanol and air over a heated platinum spiral to produce formaldehyde. This developed into the method by which most formaldehyde is produced today through the oxidation of methanol with air using a metal catalyst, making it a relatively cheap substance to manufacture in large quantities.
[edit] What materials contain formaldehyde?
Wood products naturally contain small, quite safe amounts of formaldehyde, as it is present in tree growth. The levels of formaldehyde become more of an issue when resins are added to wood and wood-based products. The different forms of formaldehyde are often used in various pressed-wood building materials and insulants, as well as in many laminates, textiles, carpets, and furniture fabrics. In some cases, the term no-added formaldehyde, or the abbreviation NAF, is used to describe products that only contain natural levels of the substance.
Formaldehyde has also been used as a fungicide, germicide, and disinfectant, used in soils, fertilisers, seed dressings, the preservation of grains, and the preparation of poultry. It may also be present in commonly used antiseptics, medicines, and cosmetics, and as a preservative in mortuaries and medical laboratories. Formaldehyde can also be present in the air, along with carbon monoxide, sulphur dioxide, nitrogen oxides, benzene, butadiene, and soot produced by vehicle exhaust fumes and tobacco smoke. There are also a number of VOC emitting products, oftren found in home that contain formaldehyde as as base element that can lead to formaldehyde off-gassing, which at low levels is generally harmless, unless increased over recommended levels.
[edit] What types of formaldehyde are there?
[edit] Urea formaldehyde
Urea formaldehyde (UF), also referred to as urea-methanal, is a thermosetting resin or polymer produced from urea and formaldehyde. It was used extensively in the production of foam insulation as well as adhesives and many other products. It is relatively cheap but does not react well to higher moisture levels, and as such, it is often used in the manufacture of interior wood products such as furniture panelling, boards, and flooring. However, it creates relatively high levels of formaldehyde off-gas, which, in an internal environment, can be toxic. As such, it has been slowly phased out in many countries due to this, and there are a number of formaldehyde-free or glue-free products available as alternatives. In terms of insulation products, urea formaldehyde foam insulation (UFFI) was banned in Canada in the 1980s and then in the US, but there is a wide variety of alternative insulants on the market that do not contain formaldehyde, as well as some blown insulants that contain alternatives, making urea formaldehyde less and less common.
[edit] Phenol formaldehyde
Phenol formaldehyde usually refers to phenol formaldehyde resin or phenolic resin, better known as bakolite, the trade product of one of the first synthetic resin plastics. This is created by using phenol and formaldehyde directly to create a thermoset. Phenol-formaldehyde resins with a formaldehyde ratio of less than one are referred to as novolaks (or novolac), which can be moulded and then cured; phenol-formaldehyde resins with a formaldehyde ratio of more than one (usually 1.5) are called resoles. Resoles are polymeric resin materials used for the glueing and bonding of construction building materials, such as exterior plywood, oriented strand board (OSB), and engineered high-pressure laminates. Novolaks are high-temperature resins used in carbon brakes, photoresists, and as a curing agent for epoxy resins. Hexamethylenetetramine is a hardener that is added to crosslink novolak resins. Phenol formaldehyde glues used in timber products tend to be harder to wear, and for external use, they also have a significantly reduced rate of off-gasing when compared to urea formaldehyde glues.
[edit] Melamine formaldehyde
Melamine formaldehyde, or melamine resin, is a hard-wearing thermoset plastic made from melamine and formaldehyde. In the 1950s and 1960s, melamine was used in fashionable tableware known as Melmac. Today, it is extensively used in the production of high-pressure laminates for kitchen surfaces (trade names Formica and Arborite), flooring, and wall panels. The name melamine often refers to furniture products with a base of particle board covered by a layer of melamine resin-soaked paper, giving a hard decorative finish suitable for furniture and kitchen cabinets.
[edit] Polyoxymethylene
Polyacetal resins, or polyoxymethylene (POM), are high-strength, stiff, hard, rigid, and stable thermoplastics used in precision parts such as hardware, hinges and mechanisms of furniture, keyboard parts,insulators, and connectors, in electronic devices such as televisions and telephones, gears, housings, and many parts in precision engineering and many other uses, from zippers to cigarette lighters.
Other materials include pentaerythritol, 1,4-butanediol, methylenebis, and a number of others.
[edit] How is formaldehyde harmful?
Public Health England has published a formaldehyde-toxicological overview in which it states some key points:
[edit] Kinetics and metabolism:
- Formaldehyde is readily absorbed following inhalation and ingestion but poorly absorbed following dermal exposure. Formaldehyde is rapidly metabolised to form at the initial site of contact; negligible amounts of inhaled or ingested formaldehyde reach systemic circulation.
- Formaldehyde is eliminated by exhalation as carbon dioxide or by urinary excretion.
[edit] Health effects of acute exposure
- Sore throat, rhinitis, nasal irritation, bronchospasm, and breathlessness are common features following exposure by inhalation.
- In severe cases, laryngeal and pulmonary oedema, pneumonitis, and acute respiratory distress syndrome may occur.
- Ingestion of formaldehyde may cause ulceration and burns, pain, nausea, vomiting, diarrhoea, gastrointestinal haemorrhage, hypotension, shock, and metabolic acidosis.
- Formaldehyde is corrosive and can cause irritation and burns to the skin and eyes; ocular exposure may result in permanent alterations in vision.
[edit] Health effects of chronic exposure
- Chronic exposure to formaldehyde causes irritation and may cause the development of histopathological lesions in the nasal mucosa.
- Formaldehyde is a known skin sensitiser in humans, causing allergic contact dermatitis.
- Formaldehyde is a human carcinogen.
[edit] How is the use of formaldehyde controlled?
As of 2016, formaldehyde was classified as a Category 1B carcinogen, meaning certain restrictions apply to it, and itsuse is only permitted for professional use and sold by a registered supplier. In the EU, regulation (EC) 2022/1181 requires a warning of ‘contains formaldehyde’ for formaldehyde-releasing substances found in cosmetic products. This was originally required for products with a concentration over 0.05%, which was then reduced to 0.001% in 2022. Formaldehyde-releasing preservatives (FRPs) are also a source of formaldehyde often used in cosmetics; these include quaternium-15, DMDM hydantoin, imidazolidinyl urea, diazolidinyl urea, polyoxymethylene urea, sodium hydroxymethylglycinate, bromopol, and glyoxal. Consumer awareness is a growing area of control, with such productsbeing increasingly labelled as formaldehyde-free.
Today detectors of volatile organic compounds (VOCs) are available to measure the levels of different VOCs in the air, in particular formaldehyde gas detectors. The Clean Air (Human Rights) Bill lists acceptable levelsof formaldehyde as being 8.6 μg/m3, internally or externally, averaging over the period of a year. 1 µg/m3 means that one cubic metre of air contains one microgram (10-6 grams) of a pollutant.
[edit] Related articles on Designing Buildings
- Air change rate.
- Air entrainment.
- Air pollution.
- Air quality hub.
- Air tightness.
- Carbon monoxide.
- Clean Air Act.
- Clean Air Act and implications for the construction industry.
- Clean Air (Human Rights) Bill;.
- Indoor air quality.
- Nitrogen.
- Types of rigid foam insulation;.
- Urea formaldehyde.
- Water vapour.
Featured articles and news
Building Safety recap January, 2026
What we missed at the end of last year, and at the start of this...
National Apprenticeship Week 2026, 9-15 Feb
Shining a light on the positive impacts for businesses, their apprentices and the wider economy alike.
Applications and benefits of acoustic flooring
From commercial to retail.
From solid to sprung and ribbed to raised.
Strengthening industry collaboration in Hong Kong
Hong Kong Institute of Construction and The Chartered Institute of Building sign Memorandum of Understanding.
A detailed description fron the experts at Cornish Lime.
IHBC planning for growth with corporate plan development
Grow with the Institute by volunteering and CP25 consultation.
Connecting ambition and action for designers and specifiers.
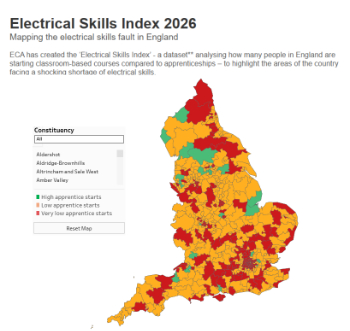
Electrical skills gap deepens as apprenticeship starts fall despite surging demand says ECA.
Built environment bodies deepen joint action on EDI
B.E.Inclusive initiative agree next phase of joint equity, diversity and inclusion (EDI) action plan.
Recognising culture as key to sustainable economic growth
Creative UK Provocation paper: Culture as Growth Infrastructure.
Futurebuild and UK Construction Week London Unite
Creating the UK’s Built Environment Super Event and over 25 other key partnerships.
Welsh and Scottish 2026 elections
Manifestos for the built environment for upcoming same May day elections.
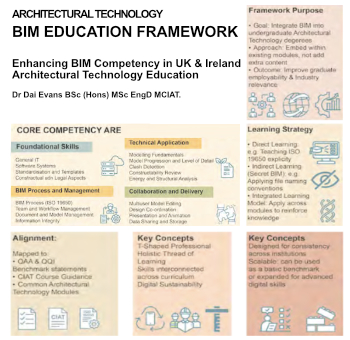
Advancing BIM education with a competency framework
“We don’t need people who can just draw in 3D. We need people who can think in data.”
Guidance notes to prepare for April ERA changes
From the Electrical Contractors' Association Employee Relations team.
Significant changes to be seen from the new ERA in 2026 and 2027, starting on 6 April 2026.
First aid in the modern workplace with St John Ambulance.
Solar panels, pitched roofs and risk of fire spread
60% increase in solar panel fires prompts tests and installation warnings.
Modernising heat networks with Heat interface unit
Why HIUs hold the key to efficiency upgrades.