Types of radioactive material (Radionuclides)
Contents |
[edit] Introduction
Radionuclides (or radioactive materials) are a class of chemicals for which the nucleus of the atom is unstable. They achieve stability through changes in the nucleus by spontaneous fission, emission of alpha particles, or conversion of neutrons to protons or the reverse.
This process is called radioactive decay or transformation, and is often followed by the release of ionizing radiation (beta particles, neutrons, or gamma rays). Alpha particles can travel only a short distance and cannot travel through skin, Beta particles can penetrate through skin, but not through an entire body, whilst Gamma radiation can go all the way through a human body. This is what makes Gamma radiation the most dangerous because it can penetrate the skin and damage the cells inside the body, whereas Apha radiation cannot.
The time the material takes to decay (along with the release of radiation) is expressed in term of half life - the time required for a quantity of a radioisotope to decay by half. A radioactive material with a short half-life will decay sooner, so it gives up its stored energy faster. That makes is more radioactive and harder to handle and work with. A radioactive material with a long half-life gives up its stored energy more slowly. That makes it less radioactive and easier to handle.
In terms of the design and construction of buildings the main concern is usually radon which can move from the ground into the home through cracks and holes in the foundation, or private well water. If it builds up in a house over many years it can cause lung cancer. Although it is a tasteless, odourless gas it is possible to use equipment to test for radon.
Some other building materials, such as granite, brick and marble, naturally contain radioactivity, including radon but the amount of radiation released from these materials is typically very low. Granite for instance contains naturally-occurring uranium, thorium and their radioactive decay products. Concrete might also contain radioactive material in small amounts when it is mixed with additives such as fly ash from coal power plants.
The final connection to building construction is in relation to power plants, particularly nuclear reactors which normally use nuclear fission, nuclear decay and nuclear fusion reactions. Currently the vast majority of electricity from nuclear power is produced by nuclear fission of uranium and plutonium in nuclear power plants.
Below are a list of different types of radioactive materials along with their uses.
[edit] Cesium
Cesium is a naturally occurring element found in rocks, soil, and dust, it is not radioactive (stable cesium). The breakdown of uranium or nuclear explosions can produce two radioactive forms that decay to non-radioactive elements.
[edit] Cobalt
Cobalt is a naturally occurring element found in rocks, soil, water, plants, and animals. Cobalt is used to produce alloys for aircraft engines, magnets, artificial joints and so on. Cobalt compounds are used for glass and ceramic colouring. Two types of radioactive cobalt exist in use, one for sterilizing medical equipment, radiation therapy, plastic manufacturing and irradiating food, the other is used in medical and scientific research.
[edit] Iodine
Iodine is a naturally occurring element in sea water and in certain rocks and sediments. There are non radioactive and radioactive forms of iodine. Iodine is used in disinfectant, skin soaps, bandages, and for purifying water. Iodine is also added to some table salt for diet. Most radioactive iodine is human-made, it changes very quickly (seconds to days) to be a non-radioactive stable element. It is used in medical disease treatment tests.
[edit] Ionizing radiation
Ionizing radiation is any one of several types of radiation given off by radioactive material, high-voltage equipment, nuclear reactions, and stars. The types that are normally important to your health are alpha particles, beta particles, x rays, and gamma rays.
[edit] Plutonium
Plutonium is a silvery white solid metal under normal conditions, produced when uranium absorbs an atomic particle and trace amounts can occur naturally. Larger amounts have been produced in nuclear reactors and trace levels can be found in the environment, from past nuclear bomb tests, in several forms called isotopes. Plutonium undergoes radioactive decay, where energy is released and a new product is formed.
[edit] Radium
Radium is a naturally occurring silvery-white radioactive metal that can exist in several forms called isotopes. Radium is formed when uranium and thorium, found in rocks and soil, break down in the environment. Radium undergoes radioactive decay, dividing into two parts - one part is radiation and the other part is a 'daughter', which is not stable, and divides again until a stable, nonradioactive daughter is formed. Alpha, beta, and gamma radiation are released during this process. Radium has been used for treating cancer, radiography of metals, combined as a neutron source for research and radiation instrument calibration. Until the 60s, it was a component of the luminous paints.
[edit] Radon
Radon is a naturally occurring radioactive gas that is odourless, tasteless. and formed from the radioactive decay of uranium. Uranium is found in small amounts in rocks and soil, it slowly breaks down to other products such as radium, which breaks down to radon. Radon also undergoes radioactive decay. It divides into two parts - one part is radiation, and the other part is a daughter. The daughter, like radium, is not stable, and it also divides into radiation and continues dividing into daughters until a stable, nonradioactive daughter is formed. During the decay process, alpha, beta, and gamma radiation are released. Radon is no longer used in the treatment of various diseases but is used to predict earthquakes, in the study of atmospheric transport, and in exploration for petroleum and uranium.
[edit] Strontium
Strontium is a non radioactive naturally occurring element found in rocks, soil, dust, coal, and oil - referred to as stable strontium or strontium which exists in four stable isotopes. Strontium compounds are used in making ceramics and glass, pyrotechnics, pigment, fluorescent lights, and medicines. Strontium can also exist as several radioactive isotopes; the most common is formed in nuclear reactors or during the explosion of nuclear weapons. Radioactive strontium generates beta particles as it decays.
[edit] Thorium
Thorium is a naturally occurring, radioactive substance. Thorium exists in combination with other minerals, such as silica in small amounts within rocks, soil, water, plants, and animals. Soil contains an average of about 6 parts of thorium per million parts of soil (6 ppm). It breaks down into a small part called alpha radiation and a larger called the decay product, this is not stable and continues to break down until a stable product is formed. During these decay processes, radioactive substances are produced which include radium and radon, these give off radiation, including alpha and beta particles, and gamma radiation. Some rocks underground contain more concentrated thorium. Thorium is usually concentrated and changed into thorium dioxide or other chemical forms. After most of the thorium is removed, the rocks are called "depleted" ore or tailings. Thorium is used to make ceramics, gas lantern mantles, aerospace metals and in nuclear reactions. Thorium can also be used as a fuel for generating nuclear energy.
[edit] Uranium
Uranium is a common naturally occurring, radioactive substance, a normal part of rocks, soil, air, and water, it occurs in the form of minerals. Uranium metal is silver-colored with a grey surface and is nearly as strong as steel. Natural uranium is a mixture of three types or isotopes. Typical concentrations in soil are a few parts per million ppm. Some rocks contain high enough concentrations to be mined - the uranium is taken out of the rocks and made into uranium chemicals or metal, the remaining sand is called mill tailings. Tailings are rich in the chemicals and radioactive materials that were not removed, such as radium and thorium. One specific isotope is useful as a fuel in powerplants and weapons, this is made by splitting natural uranium into two portions. The fuel portion more of the specific isotope is called enriched uranium, the leftover portion with less is called depleted uranium, or DU.
For further information and general guidance seek professional help and advice as well as visiting the Institute of Hazardous Materials Management (IHMM) or the Agency for Toxic Substances and Disease Registry (ATSDR)
[edit] Related articles on Designing Buildings
- Chernobyl New Safe Confinement.
- Generation nuclear.
- High Speed 2 (HS2).
- Infrastructure and Projects Authority.
- Mitigating the Delay Risk in Power Plant Projects.
- Methane and other gasses from the ground.
- National Infrastructure Plan.
- Nationally Significant Infrastructure Projects.
- Planning and managing Hinkley Point C.
- Renewable energy.
- Wind energy.
- Radon protection for new domestic extensions and conservatories with solid concrete ground floors (GG 73 revised).
- Radon: Guidance on protective measures for new buildings BR 211
- Radon protection for new dwellings GG 74.
- Radon protection for new large buildings GG 75.
- Radon.
- Radon: Guidance on protective measures for new buildings BR 211.
Featured articles and news
Spring Statement 2025 with reactions from industry
Confirming previously announced funding, and welfare changes amid adjusted growth forecast.
Scottish Government responds to Grenfell report
As fund for unsafe cladding assessments is launched.
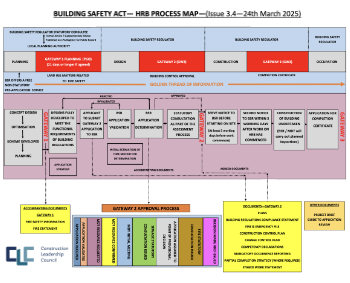
CLC and BSR process map for HRB approvals
One of the initial outputs of their weekly BSR meetings.
Architects Academy at an insulation manufacturing facility
Programme of technical engagement for aspiring designers.
Building Safety Levy technical consultation response
Details of the planned levy now due in 2026.
Great British Energy install solar on school and NHS sites
200 schools and 200 NHS sites to get solar systems, as first project of the newly formed government initiative.
600 million for 60,000 more skilled construction workers
Announced by Treasury ahead of the Spring Statement.
The restoration of the novelist’s birthplace in Eastwood.
Life Critical Fire Safety External Wall System LCFS EWS
Breaking down what is meant by this now often used term.
PAC report on the Remediation of Dangerous Cladding
Recommendations on workforce, transparency, support, insurance, funding, fraud and mismanagement.
New towns, expanded settlements and housing delivery
Modular inquiry asks if new towns and expanded settlements are an effective means of delivering housing.
Building Engineering Business Survey Q1 2025
Survey shows growth remains flat as skill shortages and volatile pricing persist.
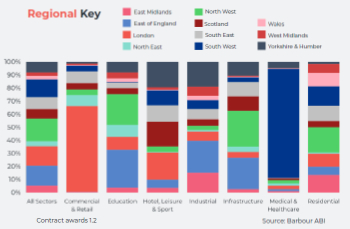
Construction contract awards remain buoyant
Infrastructure up but residential struggles.
Warm Homes Plan and existing energy bill support policies
Breaking down what existing policies are and what they do.
A dynamic brand built for impact stitched into BSRIA’s building fabric.