Fertilizer groundwater pollution
[edit] Introduction
With the outbreak of the Second World War food security became a major issue in Britain, with the government encouraging farmers to increase production. This policy was continued after the war with the 1947 Agriculture Act and, upon joining the Europe Community, the 1973 Common Agricultural Policy, both of which provided incentives for farmers to increase production.
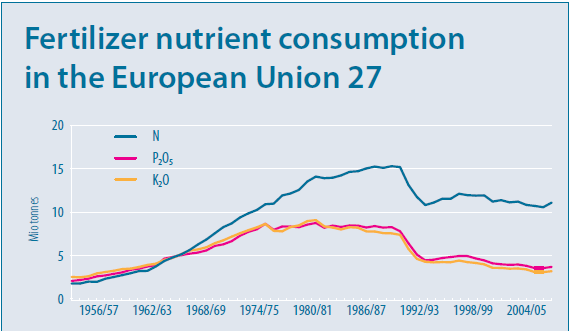
One of the biggest contributors to this increase in production was the use of fertiliser nitrogen, which in the UK increased from 200,000 tonnes/year in the mid-forties to approximately 1,580,000 tonnes in 1985. This increasing trend was matched across most of Western Europe, as shown in figure 1, and has resulted in the problems with groundwater pollution. (Department of the Environment, 1986)
Figure 1: Fertiliser nutrient consumption in Europe (European Commission, 2010)
[edit] The problem with fertilisers
The majority of fertilisers used contain three main constituents; Nitrates, Phosphates and Potassium, which are all essential to the growth of plants (ncagr, 2012) . The majority the nitrogen is in the form of ammonium nitrate (Isherwood, n.d.).
Although phosphates are the limiting nutrient in causing eutrophication in open water (MAFF, 1993), it is the nitrates that cause the main problem when they leach into groundwater due to its high solubility and associated health risks (European Commission, n.d.).
The health risk from nitrates comes from the fact that they break down into nitrites after they have been ingested through drinking water (Hill, 1991). The main risk is to young infants, who can develop methaemoglobinaemia, also known as blue-baby syndrome, which causes the red blood cells to lose their oxygen carrying capacity (Hill, 1991). While methaemoglobinaemia is not a serious risk in adults due to their proportionally lower fluid intake, there is a theoretical risk of stomach cancer from the reduction of nitrates into carcinogenic nitrosamines once consumed, although this is yet to be conclusively proven (Hill, 1991). In 1970, the World Health Organisation (WHO) concluded that 100 mg/l was harmful for human health (Elworthy, 1994).
Post-1945, along with the increased use of fertilisers, farmers began ploughing larger areas of previously unused grassland causing the oxidisation of nitrogen in the organic matter of the soil into nitrates. This meant that by 1970 there was an increase in the concentration of nitrates in groundwater across Europe.
There was a delay in the time it took for a noticeable amount of nitrates to show in the groundwater due the process of nitrate leaching. The rate of movement of nitrates to the groundwater is dependent on the thickness and material properties of the unsaturated zone between the soil and the water table. In some rock types such as chalk it can be as slow as 1m/year through the rock matrix. Thick unsaturated layers meant it was years before noticeable levels of nitrates began to accumulate. (Downing, 1998)
[edit] Preventative policy
The European Community decided to take action on the growing scientific evidence for the negative effects of nitrates with the 1980 Drinking Water Directive outlining the requirement for drinking water to contain concentrations less than 50mg/l, with a guide value of 25mg/l (OECD, 1986). The baseline for Nitrates occurring in groundwater is less than 10mg/l (European Environment Agency, 2004).
However, by 1989, 1% of the UK population were still receiving drinking water which did not comply with the original directive (Downing, 1998), with 154 sources exceeding the limit. This increased to 192 in 1990 (MAFF, 1993).
To change this, the original directive was followed up in 1991 with the EC Nitrates Directive, which to the present day remains the most important legislation across Europe (MAFF, 1993). The Nitrate Directive specifically targets the problem of nitrates polluting groundwater from agricultural sources. It aims to achieve this by reducing the amount of nitrate entering the water by good farming practices (European Commission, 2011). About 60% of nitrates in English waters come from agricultural practices (DEFRA, 2011).
The Directive set out several stages to its implementation. Firstly, each country identified ground-waters polluted or threatened with nitrate pollution. Next, Nitrate Vulnerable Zones (NVZs) were designated to those areas of land which drain into the polluted waters (European Commission, 2011).
The Directive set out a code of good agricultural practice that was voluntary for farmers unless they were within a NVZ where it was compulsory. The code limited the time fertilisers can be applied to land so that nitrates are available only when plants need it. Also, it limited where fertilisers can be applied near open water courses to reduce entry, and prescribes the use of soil winter cover crops to avoid leaching in the wetter part of the year. (DEFRA, 2002a)
As part of the directive each member nation was required to draw up action plans for farmers within NVZs (DEFRA, 2002a). These action plans were the mechanism for implementing the compulsory code of practice and also other stricter measures, for example, restricting sources of nitrate such as the amount of natural manure the farmers can apply to 170 kg/hectare/year. (DEFRA, 2002b)
There is a review process once every four years when measurements are taken on nitrate concentrations to assess the action programmes effects and whether to revise the locations of NVZs. (European Commission, 2011)
[edit] Impact of legislation
The way the Nitrate Directive has been implemented has varied across the countries of the EU and so too has its impact. For instance, some countries such as Belgium have decided to make all their territory abide by the rules of NVZs (Downing, 1998).
In the report of the 2004-2007 period, 15% of the 31,000 groundwater sampling stations across the EU gave a nitrate concentration of more than the limit of 50mg/l (Environment Agency, 2004) , but 66% gave a reading of below 25mg/l (European Commission, 2010). These figures show a generally improving trend across Europe. However, after the last review process implemented in January 2009, 62% of England is within a NVZ (Environment Agency, 2011). It is hoped that pollution will fall in these regions although due to the slow nature of groundwater recharge this is likely to take time.
Supporting the Nitrate Directive, the 2000 Water Framework Directive and the 2006 Groundwater Directive both legislate on nitrates aiming to reduce pollution through “clean status” objectives by 2015. (European Commission, 2008)
[edit] The problem with chalk
The nitrate problem is particularly challenging in areas where the groundwater aquifer is made of chalk. Most of the south of Britain (Figure 2) has a chalk aquifer including the London Basin (Environment, Dep of, 1988). These chalk aquifers are the most important in Britain with 60% of all groundwater coming from them (Jackson et al., 2008). Also, in the South East of England some areas use groundwater for as much as 74% of their public water (Figure 3) (Environment Agency, 2004). Chalk aquifers also provide a lot of groundwater in Northern France including the Paris basin (Baran N., 2008).
The problem stems from chalk having a long “lag-time” which means that it can be as long as 40 years before the nitrate leached from the soil affects groundwater Environment, Dep of, 1988) .
Figure 2 : Map of main aquifers in England and Wales (Environment Agency, 2004)
Figure 3 : Public Water supplied by groundwater in the UK (Environment Agency, 2004)
Chalk is a calcareous clay and un-fissured has a low permeability (Environment, Dep of, 1988). While its inter-granular porosity is 30-40%, its pore openings are between 0.2 and 2 μm which prevents water from draining out of the chalk matrix itself (Baran N., 2008), giving it a high storability of nitrates.
However, the chalk aquifers in Southeast England have well developed fissures within them, between 0.5 to 2% of their total volume, giving them a higher permeability (Environment, Dep of, 1988). These fractures are only utilised during high infiltration events, meaning the majority of the time rainfall is mostly accommodated by the matrix (Jackson et al., 2008).
The implication of chalks dual-porosity with water able to travel rapidly through the fissures and slowly through the matrix, gives the aquifers a unique hydrogeological character. Chalk aquifers are not expected to achieve the objectives of the Water Framework Directive by 2015 (Jackson et al., 2008). The BGS (2011) states that it will be decades before the peak nitrate concentrations resulting from large historical inputs reach the saturated zone and enter the water supply, drawing out the problem for a long period of time - the “nitrate time bomb”.
[edit] Modelling the flow through aquifers
Environmental engineers simulate how nitrates work their way into the groundwater once applied in fertiliser so that the impacts of land use management policies can be assessed. To do this they use catchment-scale models, some of which are based on statistical data and others based on physical processes.
The ones used most often are simplified, conceptual versions of physics-based models because it is often difficult and expensive to obtain all the data required to calibrate full physics-based models and the statistical models are not adaptable enough to simulate different scenarios. An example of such a model is the Integrated Catchment Nitrogen model (INCA-N), which has been used in the Lambourn and Pang river-systems in Berkshire (Jackson et al., 2008).
There are specialised models for chalk aquifers due to the complexity of the medium and the variance in time and space of the thickness of the unsaturated zone. One such model is INCA-Chalk, which is based on INCA-N and in some cases can predict nitrate concentrations very well.
However, physical models in general suffer from inaccurate historical application data and an incomplete understanding of the physical processes which occur. Using INCA-Chalk on the Lambourn catchment, Jackson et al. (2008) predict that even in the best case scenario of no more nitrates being applied, the historical application in that region will mean concentrations do not decrease until 2060.
Modelling is currently influencing decisions to make exceptions for chalk aquifer regions which will not reach the 2015 targets and increase investment in in-situ aquifer treatment as a solution to the ineffectiveness of land management with chalk. (Jackson et al., 2008)
[edit] Treatment of problematic water
There are many different methods of removing nitrates from water, the most common of which in the UK is blending a high nitrate source with a low one (Harris, 1998). This is also the cheapest method providing that the low nitrate source is not far away, however as most high nitrate sources tend to be grouped together it can be unviable.
One option is to use surface water, but it is often of poorer quality and requires costly treatment. Water companies prefer water which comes from confined aquifers as they are generally very low in nitrates. (Environment, Dep of, 1988).
An alternative treatment is storage of the high nitrate water in reservoirs, using microbiological de-nitrification processes to reduce concentration. This method can be very effective, however it has several disadvantages relating to its susceptibility to algal blooms and the required retention time restricting its provision in areas with high demand. (OECD, 1986)
Lesser used methods of de-nitrification include; ion exchange, reverse osmosis, electro-dialysis and chemical reduction. It is most usual for companies to use ion exchange although they are all effective, however in general they do not adopt these approaches due to the associated costs. (Elyanow & Persechino, 2005)
Other methods include managing the rate of extraction from boreholes and the depth and location at which water is taken from the aquifer. It can be the case that slower pumping at a deeper depth in the aquifer produces lower concentrations. Also, by undertaking a hydrogeological study when looking to establish a new borehole, a lower nitrate source can be found, although this can come with extra costs if it is difficult to reach or if it contains unwanted minerals as confined aquifers commonly do. (OECD, 1986)
As well as treating the water after it has been extracted there is a method for reducing nitrate concentrations in-situ, although this is still yet to be successfully applied at a large scale. The method involves taking advantage of the natural de-nitrification processes that take place in aquifers, with microbes breaking down nitrates in place of oxygen as they respire under anaerobic conditions.
One idea is to introduce an organic carbon feed to stimulate this process and it is hoped it may provide an inexpensive method of nitrate removal in the future. Another experimental method is to instal boreholes lined with vegetation and then circulate water from contaminated aquifers to reduce the oxygen content. (Department of the Environment, 1986; Jackson et al., 2008)
There have also been experiments with applying nitrification inhibitors to suppress the transformation of the ammonium nitrate in fertilisers into nitrate in the soil and therefore reduce nitrate leaching (MAFF, 1993).
[edit] Who pays for the de-nitrification?
Due to the diffuse nature of nitrate pollution it can be difficult to assess who should pay for its treatment. Water companies argue that the problem is caused by the agricultural industry and in accordance with the principal that the polluter should pay (PPP) it is they who they should pay for the remediation action like any other industrial polluter.
However, the agricultural industry says that they are already complying with the polluter pays principal by adhering to the European Directives which incur costs and they should not be penalised for following government policy on increasing crop production over the last forty years. (Department of the Environment, 1986)
The authorities can impose fines on farmers for failing to comply with the legislation, but it is often difficult to accurately identify polluters using modelling techniques. The amount of pollution any one individual has caused can be difficult to quantify due to the variability of the amount of nitrate leached into the soil relative to the amount of fertiliser applied. (Department of the Environment, 1986)
Currently, the water companies pay for treatment and pass the costs on to their customers. For example, Cambridge Water plans on investing £7.84m on nitrate removal plants between 2010 and 2015 which, with other treatment costs, will be passed on to its users through increases of 7.3% on the average customer’s yearly bill (Cambridge Water, 2009).
[edit] Conclusion
To conclude, the issue of nitrate pollution is a challenging one for environmental engineers because of the long length of time associated with remediation and the health risk it poses. A lot of progress has been made over the last 20 to 30 years, with the problem benefiting greatly from robust European legislation and new understanding in the fields of hydrogeology and contaminant dispersion.
There is still work to be done in the area with continuing research into new techniques of treatment and modelling, however, there has been significant progress in reductions of nitrate concentrations under the current policies with the majority of groundwater sources moving towards lower concentrations.
[edit] Related articles on Designing Buildings Wiki
- Biosolids.
- Brownfield land.
- Chalk aquifer.
- Contaminated land.
- Deleterious materials.
- Diffuse pollution.
- Environmental Policy.
- Groundwater.
- Groundwater control in urban areas.
- Gypsum.
- Pollution.
- Sustainability.
- Sustainable Urban Drainage Systems.
[edit] External references
- Baran N., L. M. a. M. C., 2008. Agricultural diffuse pollution in a chalk aquifer (Trois Fontaines,France): Influence of pesticide properties and hydrodynamic constraints. Journal of Hydrology 358, Issue 1-2, pp. 56-69.
- BGS, 2011b. British Geological Society- Nitrate Fluctuation. [Online] Available at: http://www.bgs.ac.uk/research/groundwater/quality/nitrate_fluctuations.html [Accessed 21 1 2012].
- BGS, 2011. British Geological Society- Predicting the arrival of peak nitrate concentrations at the water table. [Online] Available at: http://www.bgs.ac.uk/research/groundwater/quality/nitrate_peaks.html [Accessed 21 1 2012].
- Cambridge Water, 2009. Meeting the challenges of the next five years and beyond. [Online] Available at: http://www.cambridge-water.co.uk/includes/asp/download_file.asp?id=71 [Accessed 23 1 2012].
- DEFRA, 2002a. Guidelines for farmers in NVZs- England. 1st ed. London: DEFRA.
- DEFRA, 2002b. Manure Planning in NVZs- England. 1st ed. London: Department for Environment, Food and Rural Affairs.
- DEFRA, 2011. Nitrates and Watercourses.
- Department of the Environment, 1986. Nitrate in the Water, London: Her Majesty's Stationary Office.
- Department of the Environment, 1986. Nitrate in the Water, London: Her Majesty's Stationary Office.
- Department of the Environment, 1988b. Assessment of Groundwater Quality in England and Wales. London: Her Majesty's Stationary Office.
- Downing, D., 1998. Groundwater - Our Hidden Asset. London: British Geological Survey.
- Elworthy, S., 1994. Farming for Drinking Water. 1st ed. Aldershot, UK: Ashgate Publishing Limited.
- Elyanow, D. & Persechino, J., 2005. Advances in Nitrate Removal.
- Environment Agency, 2004. The state of groundwater in England and Wales, Bristol: Environment Agency.
- Environment Agency, 2011. Nitrate Vulnerable Zones (NVZs).
- Environment, Dep of, 1988. The Nitrate Issue, London: Her Majesty's Stationary Office.
- European Commission, 2010. The EU Nitrates Directive. [Online] Available at: http://ec.europa.eu/environment/pubs/pdf/factsheets/nitrates.pdf [Accessed 22 1 2012].
- European Commission, 2008. Groundwater protection in Europe.
- European Commission, 2011. Implementation of nitrates Directive. [Online] Available at: http://ec.europa.eu/environment/water/water-nitrates/index_en.html [Accessed 22 1 2012].
- European Commission, n.d.. Nitrogen in Agriculture.
- European Environment Agency, 2004. Nitrate in Groundwater. [Online] Available at: http://www.eea.europa.eu/data-and-maps/indicators/nitrate-in-groundwater-1#toc-2 [Accessed 21 1 2012].
- Harris, R., 1998. Protection of groundwater quality in the UK: present controls and future issues. In: Groundwater Contaminants and their Migration. London: The Geological Society, pp. 1-14.
- Hill, M., 1991. Nitrates and Nitrites in Food and Water. Cambridge: Woodhead Publishing.
- Isherwood, K., n.d.. Fertilizer Use in Western Europe: Types and Amount. [Online] Available at: http://www.eolss.net/Sample-Chapters/C10/E5-24-08-03B.pdf [Accessed 21 1 2012].
- Jackson&al., B., 2008. Nitrate transport in Chalk catchments: monitoring, modelling and policy implications. Environmental Science & Policy, 11(2), pp. 125-135.
- MAFF, 1993. Solving The Nitrate Problem:Progress in Research and Development. London: MAFF Publications.
- ncagr, 2012. Plant Nutrients. [Online] Available at: http://www.ncagr.gov/cyber/kidswrld/plant/nutrient.htm
- OECD, 1986. Water Pollution by Fertilizers and Pesticides. 1st ed. Paris, France: OECD Publication.
- Wheater, H., 2006. Understanding Our Groundwater Systems. [Online] Available at: http://www.groundwateruk.org/downloads/Howard%20Wheater.pdf [Accessed 21 1 2012].
Featured articles and news
Amendment to the GB Energy Bill welcomed by ECA
Move prevents nationally-owned energy company from investing in solar panels produced by modern slavery.
Gregor Harvie argues that AI is state-sanctioned theft of IP.
Heat pumps, vehicle chargers and heating appliances must be sold with smart functionality.
Experimental AI housing target help for councils
Experimental AI could help councils meet housing targets by digitising records.
New-style degrees set for reformed ARB accreditation
Following the ARB Tomorrow's Architects competency outcomes for Architects.
BSRIA Occupant Wellbeing survey BOW
Occupant satisfaction and wellbeing tool inc. physical environment, indoor facilities, functionality and accessibility.
Preserving, waterproofing and decorating buildings.
Many resources for visitors aswell as new features for members.
Using technology to empower communities
The Community data platform; capturing the DNA of a place and fostering participation, for better design.
Heat pump and wind turbine sound calculations for PDRs
MCS publish updated sound calculation standards for permitted development installations.
Homes England creates largest housing-led site in the North
Successful, 34 hectare land acquisition with the residential allocation now completed.
Scottish apprenticeship training proposals
General support although better accountability and transparency is sought.
The history of building regulations
A story of belated action in response to crisis.
Moisture, fire safety and emerging trends in living walls
How wet is your wall?
Current policy explained and newly published consultation by the UK and Welsh Governments.
British architecture 1919–39. Book review.
Conservation of listed prefabs in Moseley.
Energy industry calls for urgent reform.