Primary non-rechargeable batteries
Contents |
[edit] Introduction
A primary battery is a non-rechargeable, single-use battery as opposed to a secondary cell that can be recharged. A primary battery converts chemical energy into electrical energy by means of an electrochemical process. The electrochemical process that occurs is spontaneous Oxidation-Reduction (redox) this is where both a reduction reaction and an oxidation reaction take place at once. The earliest electrochemical cells were called wet cells as electrodes were submerged in a solution, later and more modern primary cells are more commonly dry cells.
A secondary or rechargeable battery acts as a galvanic cell when it is discharging, as it is converting chemical energy to electrical energy in a redox reaction but acts as an electrolytic cell when it is being charged as it is converting electrical energy to chemical energy. Electrolysis is the process by which ionic substances are broken down into simpler substances when an electric current is passed through them, thus recharging the battery's electrical potential.
In a redox reaction, electrons accumulate on the oxidation electrode (anode) and provide a negative potential, whilst a reduction process occurs at the reduction electrode (cathode), where a positive potential develops. The two electrodes are connected (called a salt bridge), so electrons flow from the oxidation electrode to the reduction electrode in an outer circuit due to the difference in potential between them, this produces an electric current.
[edit] Development
This type of primary cell where chemical energy produced in a redox reaction is converted to electrical energy is called either a galvanic or a voltaic cell and normally a wet cell. It is so-called firstly after the scientist Luigi Galvani (1737 – 1798) the pioneer of bioelectromagnetics who discovered that a frog's leg contracts when two different metals in contact touch different parts of the muscle, he initially named this animal electricity. Alessandro Volta later created the same effect using non-biological materials to challenge the animal electricity theory with his own metal-metal contact electricity theory, he is attributed as the inventor of the first electrical battery and this approach formed the basis of the modern-day primary battery that is still in use today.
In 1836 John Frederic Daniell, a British chemist and meteorologist, invented an improved primary cell called the Daniell cell. It consisted of an earthenware container filled with sulfuric acid and a zinc electrode immersed into a copper pot filled with a copper sulfate solution. This cell, which improved on other cells was itself improved on by the gravity cell invented in the 1860s by Frenchman Callaud.
The general-purpose primary zinc-carbon cell as is commonly used today stemmed from what was known as known as the Leclanché or dry cell, after the French engineer Georges Leclanché who invented it in 1866. This battery comprised a zinc alloy sheet containing small amounts of lead, cadmium, and mercury, a saturated aqueous solution of ammonium chloride, and impure manganese dioxide blended with carbon black and electrolyte formed around an electrode. It remains the basic design of the zinc-carbon dry cells available today with variations such as the zinc chloride battery.
Alkaline as opposed acid-based batteries were first developed by Waldemar Jungner in 1899, and, also separately by Thomas Edison in 1901. Showing greater safety and power potential, the Canadian engineer Lewis Urry invented the modern alkaline dry battery in the 1950s, which uses a zinc manganese dioxide.
[edit] Current use
Today alkaline batteries are the most common type of batteries in the world because of their stable, long-lasting, mobile performance, but zinc-carbon batteries are also still used as they remain cheaper. These come in various sizes with various voltages but AAA, AA, A-C batteries for example can be found in many small household appliances. Button/coin or watch cells often use the same components but are small enough to fit in smaller devices.
[edit] Related articles on Designing Buildings
Featured articles and news
Gregor Harvie argues that AI is state-sanctioned theft of IP.
Preserving, waterproofing and decorating buildings.
Many resources for visitors aswell as new features for members.
Using technology to empower communities
The Community data platform; capturing the DNA of a place and fostering participation, for better design.
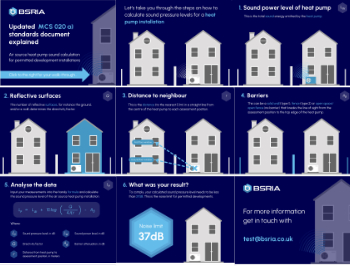
Heat pump and wind turbine sound calculations for PDRs
MCS publish updated sound calculation standards for permitted development installations.
Homes England creates largest housing-led site in the North
Successful, 34 hectare land acquisition with the residential allocation now completed.
Scottish apprenticeship training proposals
General support although better accountability and transparency is sought.
The history of building regulations
A story of belated action in response to crisis.
Moisture, fire safety and emerging trends in living walls
How wet is your wall?
Current policy explained and newly published consultation by the UK and Welsh Governments.
British architecture 1919–39. Book review.
Conservation of listed prefabs in Moseley.
Energy industry calls for urgent reform.
Heritage staff wellbeing at work survey.
A five minute introduction.
50th Golden anniversary ECA Edmundson apprentice award
Showcasing the very best electrotechnical and engineering services for half a century.
Welsh government consults on HRBs and reg changes
Seeking feedback on a new regulatory regime and a broad range of issues.