Electrochemistry
Electrochemistry is a branch of chemistry concerned with electric currents in relation to chemical reactions. Certain spontaneous chemical reactions can generate useful electric currents, whilst other chemical reactions can be forced to proceed by an electric current. Electrochemistry is the basic science behind standard batteries which are also called electrochemical cells.
Electrochemistry is also the basis of many everyday household chemical products. Bleach is made from chlorine and caustic soda, which are the products of brine electrolysis and can be made directly with an electrochemical cell. Chlorine is used to treat pools as well as drinking water, and is also an ingredient in PVC. Many cleaning agents, detergents, soaps and even paper are made or treated with the caustic soda, which is a product of brine-electrolysis.
Electrochemistry is also used to make aluminium, as it is the only economically practical way to produce the metal from its ore. Other common metals such as copper, zinc, silver and lead, are refined or purified by electrochemical processes. Many of these metals may need protection from unwanted corrosion, and this can be achieved by applying a corrosion resistant metal coating or in the case of anodising an integrated substrate. In most cases this is carried out by a process called electroplating, such as with chrome, gold or silver plating, Electroforming is where whole items are created by an electrodeposition process.
Rust in metals is the result an anodic reaction which itself is the mechanism of electrochemical corrosion, where the metal forming the anode dissolves in the electrolyte in the form of positively charged ions. There can be around 6 different types of electrochemical reactions that occur when metals become corroded, so electrochemistry is related to both the problem and the solution.
[edit] Related articles on Designing Buildings
- Brass.
- Brittle fracture.
- Corrosion coupons.
- Corrosion inhibitor.
- Corrosion resistance.
- Corrosion resistant alloy CRA.
- Crevice corrosion.
- Deterioration.
- Failure of cast iron beams.
- Galvanised steel.
- Galvanic corrosion.
- Graphitisation.
- Guidance for construction quality management professionals: Structural Steelwork.
- Hydrogen embrittlement.
- Iron.
- Marine corrosion.
- Microbiologically influenced corrosion.
- Pitting.
- Rust.
- Steel.
- Stainless steel.
- Types of metal.
- Types of steel.
- Under-deposit corrosion.
Featured articles and news
Gregor Harvie argues that AI is state-sanctioned theft of IP.
Many resources for visitors aswell as new features for members.
Using technology to empower communities
The Community data platform; capturing the DNA of a place and fostering participation, for better design.
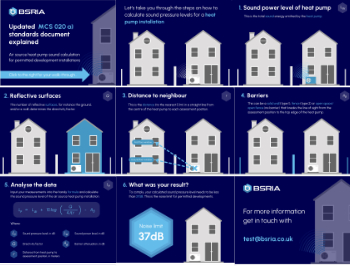
Heat pump and wind turbine sound calculations for PDRs
MCS publish updated sound calculation standards for permitted development installations.
Homes England creates largest housing-led site in the North
Successful, 34 hectare land acquisition with the residential allocation now completed.
Scottish apprenticeship training proposals
General support although better accountability and transparency is sought.
The history of building regulations
A story of belated action in response to crisis.
Moisture, fire safety and emerging trends in living walls
How wet is your wall?
Current policy explained and newly published consultation by the UK and Welsh Governments.
British architecture 1919–39. Book review.
Conservation of listed prefabs in Moseley.
Energy industry calls for urgent reform.
Heritage staff wellbeing at work survey.
A five minute introduction.
50th Golden anniversary ECA Edmundson apprentice award
Showcasing the very best electrotechnical and engineering services for half a century.
Welsh government consults on HRBs and reg changes
Seeking feedback on a new regulatory regime and a broad range of issues.
CIOB Client Guide (2nd edition) March 2025
Free download covering statutory dutyholder roles under the Building Safety Act and much more.