Corrosion resistance
Contents |
[edit] Introduction
Corrosion is the gradual destruction of materials (often metals) due to natural processes such as oxidation. It occurs when materials are exposed to the environment and are attacked by liquids or gasses whose actions instigate chemical reactions.
Corrosion resistance describes the ease with which materials react to potentially corrosive conditions. Corrosion resistant materials are generally able to withstand deterioration and chemical breakdown.
[edit] Calculating corrosion resistance rates
It is possible to calculate the corrosion resistance rate of materials in terms of weight loss or thickness loss in mils (0.001 inch) per year (mpy) or millimetres per year (mm/yr). The most accurate way to determine this rate is when the surface of the material has corroded uniformly across the area that has been exposed. Corrosion resistance rates are typically measured in testing environments where conditions such as pressure, temperature and air velocity are controlled.
[edit] Resistant metals and alloys
Certain metals have better natural resistance to corrosion, this is why metals such as gold and platinum are almost always found naturally in pure form. There are also some metals (such as steel, zinc and copper) where the oxide that forms (sometimes called a ‘patina’) acts to seal the surface and produce a protective corrosion resistant barrier.
There are some corrosion resistant alloys (CRAs) that have better inherent corrosion resistance than pure metals. Examples of CRAs include:
- Chrome
- Cobalt
- Iron
- Nickel
- Stainless steel
- Titanium
[edit] Metals and alloys susceptible to corrosion
Other metals are less stable and require corrosion resistant treatments such as the application of paints or coatings that can prevent or delay the onset of the process. Other corrosion resistance methods (such as galvanising and anodising) can be applied early in the manufacturing process.
Some metal alloys corrode simply on exposure to air, but the process can be exacerbated by the presence of heat and certain substances such as sulphates and other acidic agents. These alloys also require the application of corrosion inhibitors.
[edit] Corrosion proofing
Corrosion proofing relates to products or structures that incorporate high corrosion resistance properties directly into the manufacturing process. Corrosion proofing can come in the form of structural coatings that stop chemical and electrochemical reactions. This term is also sometimes used as a marketing term to describe products that are intended to provide permanent protection against corrosion.
[edit] Related articles on Designing Buildings
Featured articles and news
Great British Energy install solar on school and NHS sites
200 schools and 200 NHS sites to get solar systems, as first project of the newly formed government initiative.
600 million for 60,000 more skilled construction workers
Announced by Treasury ahead of the Spring Statement.
The restoration of the novelist’s birthplace in Eastwood.
Life Critical Fire Safety External Wall System LCFS EWS
Breaking down what is meant by this now often used term.
PAC report on the Remediation of Dangerous Cladding
Recommendations on workforce, transparency, support, insurance, funding, fraud and mismanagement.
New towns, expanded settlements and housing delivery
Modular inquiry asks if new towns and expanded settlements are an effective means of delivering housing.
Building Engineering Business Survey Q1 2025
Survey shows growth remains flat as skill shortages and volatile pricing persist.
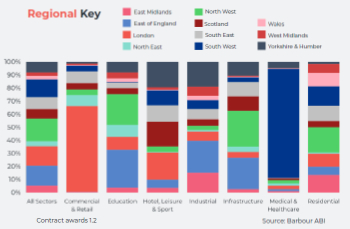
Construction contract awards remain buoyant
Infrastructure up but residential struggles.
Home builders call for suspension of Building Safety Levy
HBF with over 100 home builders write to the Chancellor.
CIOB Apprentice of the Year 2024/2025
CIOB names James Monk a quantity surveyor from Cambridge as the winner.
Warm Homes Plan and existing energy bill support policies
Breaking down what existing policies are and what they do.
Treasury responds to sector submission on Warm Homes
Trade associations call on Government to make good on manifesto pledge for the upgrading of 5 million homes.
A tour through Robotic Installation Systems for Elevators, Innovation Labs, MetaCore and PORT tech.
A dynamic brand built for impact stitched into BSRIA’s building fabric.
BS 9991:2024 and the recently published CLC advisory note
Fire safety in the design, management and use of residential buildings. Code of practice.